Chemical name: lithium carbonate
CAS accession number: 554-13-2
Specifications:
Appearance:colorless monoclinic crystal or white powder
Chemical formula:Li2CO3
Molecular weight:73.89
Density g/cm3 :2.11
Melting point ℃ :720
Boiling point ℃ :1342
Li2CO3 %:99.62
H2O %:0.11
Na % :0.015
K % :0.0005
Ca % :0.003
Mg % :0.001
Si %: 0.0005
Fe %: 0.001
Cu % :0.0002
Pb % :0.0001
Ni % :0.0005
Mn % :0.0002
Zn %: 0.0001
Al % :0.0001
Cl- % :0.0022
SO42+ % :0.015

◆Nature:white monoclinic powder is slightly soluble in water, insoluble in alcohol and soluble in acid. It is stable to heat below 600 ℃ and partially decomposes into lithium oxide and carbon dioxide at 618 ℃.
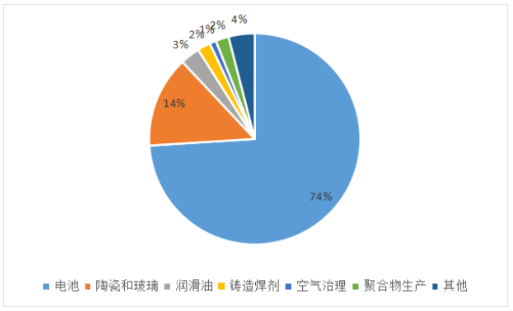
◆Application: Battery grade lithium carbonate is mainly used to prepare lithium cobaltate, lithium manganate, ternary materials and lithium iron phosphate and other lithium ion battery cathode materials;
can be used in the manufacture of lithium compounds, enamel and glass. It is a raw material for the preparation of lithium compounds and metal lithium. It can be used as an electrolytic bath additive for aluminum smelting;
are widely used in glass, ceramics, medicine, food and other industries, and can also be used in synthetic rubber, dyes, semiconductors,military and defense industries, etc;
can also be used to treat manic psychosis, make sedatives, etc.
◆Storage method: Store in a ventilated and dry place, pay attention to prevent rain and water immersion;
Do not contact with acid articles.
Lithium carbonate, lithium carbonate, molecular formula Li2CO3, molecular weight 73.891, melting point 720℃, boiling point 1342℃, density 2.11g/cm3, the appearance of white powder. It is an inorganic compound, colorless monoclinic crystal, slightly soluble in water, dilute acid, insoluble in ethanol and acetone. Used as raw materials for ceramics, glass, ferrite, etc., component silver spray pulp, etc., used in medicine to treat mental depression.
Lithium is the lightest metal in nature, with strong electrochemical activity, high specific heat capacity, large conductivity and other physical and chemical properties, widely used in the field of lithium-ion batteries and other new energy materials, known as "industrial MSG"; It is one of the elements with the most potential for application and development in the 21st century, especially with the rapid development of the new energy industry, lithium has become one of the strategic metal elements to support the realization of the dual carbon goal because of its excellent characteristics of "energy metal".

Production technology
The raw materials of lithium carbonate are generally spodumene, lepidolite and salt lake brine. In recent years, China is actively developing lithium resources in salt lakes, but the magnesium content in salt lake brine is very high, and magnesium and lithium are difficult to separate, so lithium ore is generally used to extract.
There are two kinds of production processes for extracting lithium from ore and extracting lithium from brine due to different raw material routes. As far as the actual situation is concerned, China's ore lithium extraction technology has great advantages in process and production capacity, and has become the current mainstream; The development of brine lithium extraction technology is relatively slow, but in recent years, Qinghai salt lake brine lithium extraction technology has made a major breakthrough, although Qinghai has a large-scale brine lithium extraction project put into operation, but due to resource and cost constraints, the future domestic lithium carbonate production will still be dominated by ore lithium extraction, and will maintain the pattern for a long time.
China is rich in spodumene mineral resources, Sichuan Province Kangding methyl Ka is the world's second largest spodumene mine, reserves up to 1,887,700 tons, lithium grade is also relatively high, easy to exploit and use. Yichun City, Jiangxi Province has the world's largest reserves of leucite mine, proved available lithium oxide resources of more than 2.5 million tons, can produce about 62.5 million tons of leucite concentrate with a lithium oxide grade of about 4%, the market potential is huge. There are also different processes due to different raw ore content and components.
(1) The production of lithium carbonate using spodumene
Because of its simple chemical composition, high lithium content and strong chemical inertia, spodumene has always been one of the main ore resources for extracting lithium.
The extraction process of lithium ore generally requires crushing and grinding to screen out high-grade lithium concentrate, and then lithium salt products are obtained under different technological conditions. According to the different lithium extraction medium, the lithium extraction process of spodumene mainly includes six categories, namely: sulfuric acid method, limestone method, sulfate method, chlorination roasting method, fluorine chemical method and soda ash pressure boiling method. Comprehensive production process, energy consumption and technical process difficulty. At present, sulfuric acid roasting method is the mainstream production process, and most of the major production enterprises also use sulfuric acid method to extract lithium.
At present, the production technology of lithium carbonate from spodumene is relatively mature. The advantages of this method are: high yield; It can be extracted from ores with low lithium content (1% ~ 1.5%); High lithium content (35g/L-55g/L) in leaching roasting solution. The disadvantage is that a considerable amount of sulfuric acid reacts with soda ash to produce low value sulfate; Adding sulfuric acid for roasting, environmental protection pressure, tail gas treatment investment and operating costs are high. Therefore, sulfuric acid method should control the amount of sulfuric acid added.
(2) Using lepidolite to produce lithium carbonate technology
Although spodumene is the main raw material for extracting lithium from ores because of its simple element composition, high lithium grade and abundant reserves, its industrial development and utilization are limited to some extent by the high cold and high altitude mining environment. Our country is also rich in lepidolite resources. Yichun, Jiangxi Province has the world's largest associated lepidolite deposit, and lepidolite contains high value rubidium and cesium. Therefore, it is of great significance to develop efficient lithium extraction process for the comprehensive utilization of lepidolite resources. Different from spodumene, lepidolite has complex chemical composition, low lithium grade, associated with about 2.14% rubidium and 0.91% cesium, and 55% of rubidium and cesium resources in China come from lepidolite. Moreover, lepiolite contains 5%-10% fluorine, which will cause lithium loss in the process of lithium extraction and affect the leaching of lithium. Therefore, it is necessary to consider the recovery of rubidium and cesium and the influence of fluorine in the development of high efficiency lithium extraction process of lepidolite.
At present, the main processes developed in China for extracting lithium from lemica include sulfate roasting, limestone sintering and sulfuric acid roasting, and the more mature sulfate roasting method is used.
The advantages of the sulfate roasting method are: the amount of slag is only 40% of the sulfuric acid roasting method; The recovery rate of lithium oxide is up to 75%. The energy consumption is low, equivalent to 35% of the limestone sintering method. The shortcomings of the process are also obvious, the amount of auxiliary materials added is large, the cost is high; The system is a sulfate system, and the by-products need to be further separated. The composition of lepidolite is complex and the process is long. Potassium, riveted, absolute and other precious metals in lepidolite were not recovered. Slag availability is an unknown quantity.
(3) Extraction of lithium from brine
In the world's proven lithium deposits, about 70% of lithium resources exist in salt lake brine. Compared with the extraction of lithium from ore, the extraction of lithium from salt lake brine has the advantages of low cost, low energy consumption and less pollution.
Salt lake brine contains a lot of sodium, potassium, boron, calcium, magnesium and other alkali metal chloride, sulfate, carbonate and borate, the chemical composition is complex. The composition of the major salt lakes in the world is different, so the extraction process of lithium in each salt lake is also different. The brine often contains a large number of magnesium ions, whose chemical properties are very similar to lithium, which will seriously interfere with the separation and purification of lithium salt. Therefore, reducing the magnesium-lithium ratio plays a crucial role in extracting lithium from brine.
Compared with foreign salt lake water quality, most of China's salt lake brine lithium resources have the characteristics of high magnesia-lithium ratio and high lithium concentration, which further increases the difficulty of lithium extraction production from salt lake brine. Lithium resources in China's salt lakes are mainly distributed in Tibet and Qinghai. Salt lake brine in Tibet is of high quality, but its salt lake resources are scattered, and Tibet's natural environment is poor and supporting infrastructure is lacking, which is not conducive to large-scale production. Most of the brine in Qinghai Salt Lake is of high magnesia-lithium ratio, so it is difficult to exploit lithium resources.
According to the characteristics of lithium content and magnesia-lithium ratio in salt lake water quality around the world, the industrial application of lithium extraction process methods mainly include precipitation, extraction, adsorption and so on. For lithium resources in brine with low magnesium-lithium ratio, carbonate precipitation is commonly used to extract lithium. By adding lime to remove Mg2+ and Ca2+ interfering ions in brine, lithium-rich solution is obtained, and further purified to obtain lithium carbonate products, such as Atacama Salt Lake in Chile and Silver Peak Salt Lake in the United States. For salt lake brines with high magnesium-lithium ratio, such as most salt lakes and Dead Sea brines in Qinghai and Tibet of China, aluminate precipitation, adsorption and extraction methods are commonly used.
Whether lithium ore or salt lake brine is used to produce lithium carbonate, there are more or less technical problems that need to be further solved and improved. The efficient development and comprehensive utilization of lithium resources is the inevitable development trend of the industry, and the production enterprises should consider the source of raw materials, transportation costs, energy consumption, waste gas, waste residue, wastewater treatment and other issues when choosing the right production process in combination with the actual situation, in order to achieve a clean, efficient and comprehensive utilization of lithium extraction process.
The downstream consumption of lithium carbonate is mainly the application of industrial grade lithium carbonate and battery grade lithium carbonate in the market.
As an important industrial chemical, industrial grade lithium carbonate is widely used in battery, metal oxide and other industries. Unlike sodium carbonate, which forms at least three hydrates, industrial-grade lithium carbonate exists only in anhydrous form. It has a very low solubility in water compared to other lithium salts. Lithium, which is isolated from the water extract of lithium ores, can take advantage of this poor solubility.
Industrial grade lithium carbonate application:
1, as a raw material reagent for the synthesis of other lithium compounds;
2, used in the production of ceramics and electrical insulation porcelain;
3. Manufacture heat-resistant ceramic coatings for combustion chambers and nozzles of jet engines;
4, as glaze, enamel, aluminum, cast iron and steel acid-resistant coating;
5, as a glass additive. The glass mixed with lithium carbonate has high strength, high gas resistance and high quality.
6, as a flux for aluminum manufacturing (used to improve the productivity of the electrolytic cell);
7. Used as a flux for welding aluminum and magnesium;
8, as part of the fireworks mixture;
9,Fuel cell;
10, as an additive in the cement industry, improve the acceleration and rapid curing process;
11, as a mood stabilizer in psychiatry, mainly used to treat depression and depression; Materials such as lithium carbonate and silicon dioxide form low-melting fluxes.
The chemical composition of battery grade lithium carbonate is higher in purity and contains fewer impurities, which can effectively ensure the performance of the battery. Battery grade lithium carbonate is mainly used to prepare lithium cobaltate, lithium manganate, ternary materials and lithium iron phosphate and other lithium ion battery cathode materials. With the continuous development of The Times, and the increasing tension of the energy situation, the use of batteries is increasing, and the market demand for lithium carbonate, the main raw material in the battery industry, is also increasing.
The market price of lithium carbonate is 175000 yuan /-550000 yuan/ton, please consult the customer service of this site for details.




