Chemical name: lithium hydroxide
CAS accession number: 1310-66-3
Specifications:
Appearance:white crystalline powder
Chemical formula: LiOH·H2O
Molecular weight :41.96
Density g/cm3: 1.46
Melting point ℃: 462℃
Boiling point ℃ :924
LiOH %: 57.21
CO2 % :0.38
Na+K %: 0.015
Fe2O3 %: 0.005
CaO %:0.02
SO42- %: 0.008
Cl- % :0.0015
HCI insoluble %: 0.001
Water insoluble % :0.005

◆Nature:white crystalline powder, soluble in water, slightly soluble in alcohol; It can absorb carbon dioxide from the air and deteriorate. It is strongly alkaline, does not burn, but is highly corrosive.
◆Storage method:is stored in a dry and clean compartment, away from fire and heat sources, to prevent direct sunlight, and the package is sealed. It should be connected with oxidants, acids, carbon dioxide,Edible chemicals are stored separately and do not mix them.
Introduction to monohydrate lithium hydroxide
Lithium hydroxide is an advanced renewable energy source, which opens up a whole new field of renewable energy. It consists of water, alkaline hydrogen and lithium metal, which reacts by mixing hydrogen and lithium metal to produce oxides of hydrogen and lithium, and then releases a large amount of energy to make it a renewable energy source.
The use of single-water lithium hydroxide has many advantages, such as protecting the environment, reducing greenhouse gas emissions and low-cost energy alternatives, helping to improve energy efficiency, reduce air pollution, reduce green exhaust gas and nitrogen oxide emissions, help to protect and improve the atmospheric environment, can replace traditional fuels, reduce harmless or harmful waste transportation, improve the distribution of electricity energy.
In addition, due to the very low greenhouse gas emissions released by the production process of water lithium hydroxide, it also has a small impact on the environment, which can effectively mitigate the threat of global warming and provide more renewable energy for the world.
Single-water lithium hydroxide can be used in a wide range of applications, including agriculture, industrial manufacturing and domestic housing, thereby reducing pollution, improving environmental quality and enhancing performance in the use of renewable energy for the world.
Therefore, single-water lithium hydroxide has broad prospects, can replace traditional energy sources, but also can promote the implementation of green development, and make due contributions to the world's energy security and sustainable development.
Material characteristics: monohydrate lithium hydroxide, white monoclinic fine crystal, spicy taste, strong alkaline. The chemical formula is LiOH.H2O, which is a widely used lithium compound and can be used to prepare other lithium salts.
The main raw material for the production of monohydrate lithium hydroxide is lithium carbonate, which is prepared by the reaction of lithium carbonate and lime milk.
Single-water lithium hydroxide is loose powder, but the fluidity is not good, according to the production of raw materials and different processes, the free water content before drying is 2% to 3%, and the free water content of the product after drying is 0.2%-0.5%.

Single water lithium hydroxide use
Lithium hydroxide is one of the most important lithium compounds, mainly used in the production of lithium based greases, can also be used in the production of other lithium compounds, is an alkaline battery electrolyte additive.
First, monohydrate lithium hydroxide is an important chemical that is widely used in the preparation of other lithium compounds, such as lithium carbonate, lithium chloride, etc. In addition, single-water lithium hydroxide can also be used to prepare lithium ion battery cathode materials, such as lithium cobaltate, lithium nickelate and so on.
Secondly, single-water lithium hydroxide can also be used for metal surface treatment. Soaking metal materials in a solution containing single-water lithium hydroxide can effectively remove surface oxides and other contaminants, making the metal surface smoother and easier to work with.
In addition, single-water lithium hydroxide also has certain catalytic properties, which can be used to catalyze organic chemical reactions, such as the reduction reaction of aldehydes and ketones, and the addition reaction of acid anhydrides.
In summary, monoaqueous lithium hydroxide is an important chemical with a wide range of uses, including the preparation of other lithium compounds, the preparation of cathode materials for lithium-ion batteries, metal surface treatment and organic chemical catalysis.

Single water lithium hydroxide production technology
Single-water lithium hydroxide production process
Spodumene + limestone → proportioning → pulping → roasting → grinding → concentration → filtration → impurity removal → evaporation → centrifugal dehydration → Inspection → Packaging → single-water lithium hydroxide finished product.
1. Limestone roasting method
Lithium hydroxide is prepared from lithium-bearing minerals by calcination of limestone. The production process includes raw material preparation, calcination, leaching, slag washing, concentration, purification and crystallization of leaching solution. The raw material preparation process is mainly to grind limestone fine, and press lithium minerals: limestone equal to 1:(3.05~3.15) and a certain calcium oxide (40%~42%) qualified raw pulp. The purpose of roasting is to put qualified raw pulp into a rotary kiln to undergo a series of physical and chemical changes at a certain temperature. Converts the lithium in the mineral into a water-soluble compound. Leaching is mainly to let the lithium compounds dissolved in water into the solution as far as possible, so as to separate with a large number of Ca,Si,Al impurities, SO42- in the leaching solution can be precipitated by Ba(OH)2, insoluble impurities are separated by filtration, and soluble impurities are washed, redissolved, recrystallized to refine the crude lithium hydroxide to meet the quality requirements of severe products. Taking the preparation of lithium hydroxide from lithiumite by calcination of limestone as an example, the process flow is shown in the following figure.
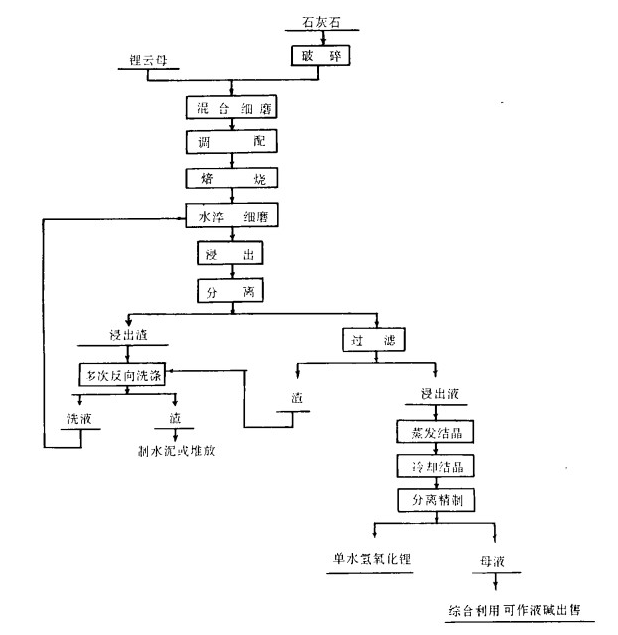
Using limestone roasting method to make single water lithium hydroxide process flow chart ↑
2. Pressure cooking method
The lithium ore is roasted separately in a rotary kiln to make it defluorinated, complete a series of physical and chemical changes, and the calcined lemica and lime are mixed at a ratio of 1 (1.0~1.2) at 150℃ with water pressure. After stirring for 1h, the following reactions occur:
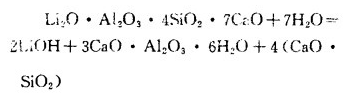
The produced lithium hydroxide enters the solution, and 3CaO·Al2O3,·6H2O and CaO·SiO2 enter the slag as the condensed phase. The soluble aluminum in the solution is removed by lime causticization, and the leaching solution is concentrated and refined in the same principle as the limestone roasting method.
3. Lithium carbonate causticization method
LiOH is obtained by the reaction of lime milk with lithium carbonate, the reaction is as follows:
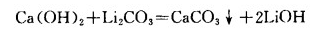
LiOH enters the solution, and calcium and carbonates form calcium carbonate into the slag. After separation, the LiOH solution was concentrated and crystallized to obtain monohydrate lithium hydroxide. Lithium carbonate causticization to produce lithium hydroxide is the main method to produce lithium hydroxide at home and abroad.
Need to buy a single water lithium hydroxide products, please consult Shanghai art letter customer service oh.

